Which Is The Best Leaving Group
Which Is The Best Leaving Group. H2N H OH A Which option correctly ranks the marked groups from best to worst leaving group. So we can identify weak bases by looking at a pKa table. AF- BCI- CI- DBr- EH2N- Which statement is true for SN2 reactions. Polarizable to stabilize the transition state.
Question 2 The best leaving group in an elimination reaction is.

Which is the best leaving group. A leaving group LG is an atom or a group of atoms that is displaced as stable species taking with it the bonding electrons. Though to be a good leaving group it should act as weak base which can accept electrons. Halides and the tosyl group -OTs are examples of commonly used leaving groups.
Next we have hydrobromic acid approximate pKa of negative nine. Best Leaving Group in Substitution. The pKa value measures the position of an equilibrium.
5 Relative rates for leaving groups k X k Br in each reaction. Become a member and unlock all. They dont want to share them with other atoms.
The best leaving groups want those electrons. In the case below tosylate is the best leaving group when ethoxide is the nucleophile but iodide and even bromide become better leaving groups in the case of the thiolate nucleophile. Specifically the large size of the bromide is able to dilute the negative charge which stabilizes the atom as the charge is not overly concentrated in a small area.
Answer N 2 is best leaving group among given. Using the acidity-substitution analogy to identify good leaving groups. The correct option is d.
Which of the following is the best leaving group. Let me write that down here. Think about why this might be true.
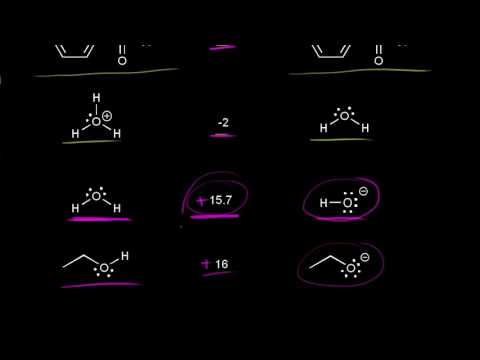
Likewise a m olecule that is neutral after leaving is generally a better leaving group than one that is negatively charged after leaving. Fluoride is the least effective leaving group among the halides because fluoride anion is the most basic. Cl - or a neutral molecule eg.
Leaving Group Ability The best leaving groups are. Which is the best leaving group in a substitution reaction of an alkyl halide from the following choices. Best leaving group are self detachable.
And since this is the conjugate based to the strongest acid this is the most stable base. Weak bases are more stable with an extra set of electrons and therefore make good leaving groups. Stable not a strong base weaker base once they have left.
Since bromide is fairly stable because of this it is able to be a leaving group. All are equally good leaving groups. Iodide which is the least basic of the four common halides F C l B r and I is the best leaving group among them.
Sulfonate structures and pKa values. This is the most stable base on the table which means that the iodide anion is an excellent leaving group because it is very stable. Good leaving groups are weak bases.
Thus a weak base is a better leaving group than a strong base. AThe rate of the reaction is dependent on the stability of a carbocation. Typically the leaving group is an anion eg.
By this logic the conjugate bases of strong acids like I Br and Cl tend to make good leaving groups. Ranking Leaving Groups Lets define a good leaving group as one that leaves easily. Then the effectiveness of a leaving group increases with the groups energetic stability after it has left.
OTO FI осон B но C OCH E А D Acid pka Acid Epka TosOH -28 H2O 1575 CH3COOH 476 CHOH 16 СА OD 8 Previous. In order for a leaving group to leave it must be able to accept electrons. 5 Relative rates for leaving groups k X k Br in each reaction.
BThe rate of reaction is dependent on just the substrate. Weak bases have strong conjugate acids. Which of the labeled groups in compound A is the best leaving group.
So we can identify weak bases by looking at a pKa table. Electron-withdrawing to polarize the carbon atom. Which of the labeled groups is the worst leaving group.
H2N H OH A Which option correctly ranks the marked groups from best to worst leaving group. CThe fastest reaction will occur with a tertiary halide. In the case below tosylate is the best leaving group when ethoxide is the nucleophile but iodide and even bromide become better leaving groups in the case of the thiolate nucleophile.
Therefore I I is the best good leaving group. The better the leaving group the more likely it is to depart. The best leaving groups will be able to stabilize the extra electrons.
PKa threshold for good leaving groups. Weak Bases are the Best Leaving Groups Now that we understand how electronegativity size and resonance affect basicity we can combine these concepts with the fact that weak bases make the best leaving groups.
This page contains many videos about which is the best leaving group. Question 2 The best leaving group in an elimination reaction is? OTO FI осон, , B но C OCH E А D Acid pka Acid Epka TosOH -2.8 H2O 15.75 CH3COOH 4.76 CHOH 16 СА OD 8 Previous. Best Leaving Group in Substitution. Which is the best leaving group in a substitution reaction of an alkyl halide from the following choices? A. F-B. Cl-C. Br-D. I-E. All are equally good leaving groups; Loading .... In the case below, tosylate is the best leaving group when ethoxide is the nucleophile, but iodide and even bromide become better leaving groups in the case of the thiolate nucleophile. [5] Relative rates for leaving groups ( k X / k Br ) in each reaction. A general rule for what makes a good leaving group is the weaker the conjugate base, the better the leaving group. In this case, halogens are going to be the best leaving groups, while compounds such as amines, hydrogen, and alkanes are going to be quite poor leaving groups.. Leaving Group Ability The best leaving groups are: • Electron-withdrawing, to polarize the carbon atom. • Stable (not a strong base, weaker base ) once they have left. • Polarizable, to stabilize the transition state. 28.
Videos of which is the best leaving group:

Duration: 3:34. Views: 1K views

Duration: 28:38. Views: 79 views

Duration: 1:01. Views: 207 views

Duration: 55:45. Views: 97 views

Duration: 1:47. Views: 9.8K views
![[hot]-3-members-of-girls'-generation-decide-to-be-leaving-the-group--snsd-at-risk-of-disbandment](https://i.ytimg.com/vi/EGEpAUVodew/hqdefault.jpg)
Duration: 2:30. Views: 21K views

Duration: 1:50. Views: 34 views

Duration: 2:56. Views: 492 views

Duration: 0:07. Views: 4.6K views

Duration: 7:45. Views: 144K views

Duration: 1:36. Views: 8K views

Duration: 4:59. Views: 4.4K views

Duration: 2:42. Views: 6.1K views

Duration: 2:06. Views: 620 views

Duration: 3:36. Views: 61K views

Duration: 10:35. Views: 6.1K views

Duration: 1:32:44. Views: 9K views

Duration: 1:04. Views: 28K views

Duration: 3:12. Views: 2.2K views

Duration: 5:32. Views: 151K views
Which of the labeled groups in compound A is the best leaving group? Which of the labeled groups is the worst leaving group? H :0: H2N H :OH A Which option correctly ranks the marked groups from best to worst leaving group?. Ranking Leaving Groups Let's define a good leaving group as one that leaves easily. Then the effectiveness of a leaving group increases with the group's energetic stability after it has left. Thus a weak base is a better leaving group than a strong base. Likewise, a m olecule that is neutral after leaving is generally a better leaving group than one that is negatively charged after leaving. Halides and the tosyl group (-OTs) are examples of commonly used leaving groups.. The best leaving groups will be able to stabilize the extra electrons. Weak bases are more stable with an extra set of electrons and therefore make good leaving groups. By this logic, the conjugate bases of strong acids (like I –, Br –, and Cl –) tend to make good leaving groups.. Using the acidity-substitution analogy to identify good leaving groups. pKa threshold for good leaving groups. Sulfonate structures and pKa values.. Ch 8 : Leaving Groups. Chapter 8: Nucleophilic Substitution. Leaving Groups. A leaving group , LG, is an atom (or a group of atoms) that is displaced as stable species taking with it the bonding electrons. Typically the leaving group is an anion ( e.g. Cl -) or a neutral molecule ( e.g. H 2 O). The better the leaving group, the more likely it is to depart.. The best leaving groups "want" those electrons. They don't want to share them with other atoms. Good leaving groups are weak bases. Weak bases have strong conjugate acids. So we can identify weak bases by looking at a pKa table. Caution: The pKa value measures the position of an equilibrium.. In the case below, tosylate is the best leaving group when ethoxide is the nucleophile, but iodide and even bromide become better leaving groups in the case of the thiolate nucleophile. [5] Relative rates for leaving groups ( k X / k Br ) in each reaction. Weak Bases are the Best Leaving Groups Now that we understand how electronegativity, size, and resonance affect basicity, we can combine these concepts with the fact that weak bases make the best leaving groups. Think about why this might be true. In order for a leaving group to leave, it must be able to accept electrons.. Answer N 2 is best leaving group among given. Best leaving group are self detachable.. Specifically, the large size of the bromide is able to dilute the negative charge, which stabilizes the atom as the charge is not overly concentrated in a small area. Since bromide is fairly stable because of this, it is able to be a leaving group.. And since this is the conjugate based to the strongest acid, this is the most stable base. Let me write that down here. This is the most stable base on the table, which means that the iodide anion is an excellent leaving group because it is very stable. Next, we have hydrobromic acid, approximate pKa of negative nine.. Which of the following is the best leaving group? A)F^- B)CI^- C)I^- D)Br^- E)H2N^- Which statement is true for SN2 reactions? A)The rate of the reaction is dependent on the stability of a carbocation. B)The rate of reaction is dependent on just the substrate. C)The fastest reaction will occur with a tertiary halide.. The halide ions are conjugate base of strong acids as they acquire negative charge during the dissociation in the aqueous solution. They can stabilize the negative charge as they have high value of....





































Posting Komentar untuk "Which Is The Best Leaving Group"