How To Determine If A Substance Is Pure Or A Mixture
How To Determine If A Substance Is Pure Or A Mixture. The components included in its composition-oxygen nitrogen carbon dioxide and so on. Generally substances can be subdivided into two groups. Decide whether a substance is chemically pure. In a pure substance each constituent does not retain its original properties.
In this animated lecture pure substances mixtures pure substances and mixtures classification of matter types of pure substances what is a pure substan.

How to determine if a substance is pure or a mixture. A pure substance produces one spot on the chromatogram an impure substance produces two or more spots A paper. Mixtures are made up of several substances that are not chemically bonded. If the mass of one component is ten times smaller than the mass of the other such a substance called a mixture.
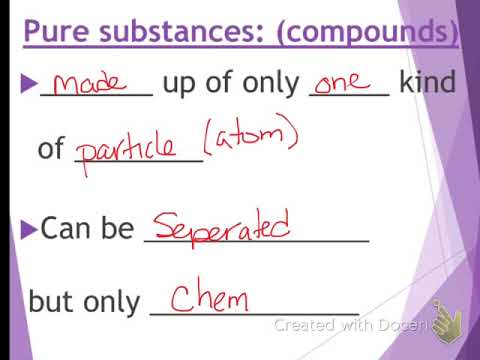
Two or more elements combined into one substance through a chemical reaction such as water form a chemical compound. Every portion of the mixture is like every other portion. A pure substance in its purest form does not contain impurities.
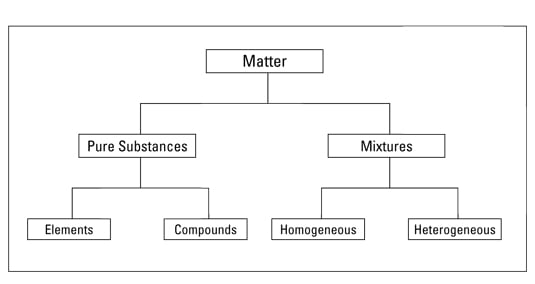
Since a mixture doesnt undergo any ionic reaction to be created it can also be. Generally substances can be subdivided into two groups. If it represents a mixture classify the mixture as homogeneous or heterogeneous.
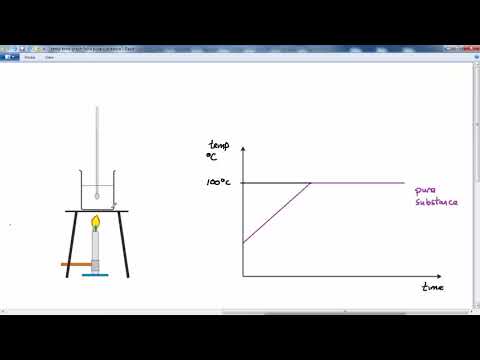
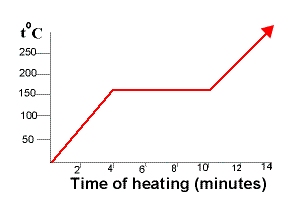
This difference is most easily seen when the temperature of a liquid is measured as it cools and freezes. The melting point is lowered. An impure substance is a type of mixture so melting points can be used to find out if a substance is pure or impure.
The components included in its composition-oxygen nitrogen carbon dioxide and so on. All pure substances have a uniform characteristic melting and boiling points. Pure substances are made of only one matter.
A pure substance has constant physical and chemical properties while mixtures have varying physical and chemical properties ie boiling point and melting point. Mixtures can be either homogeneous or heterogeneous. If a substance can be separated into its elements it is a compound.
A pure solid has a constantfixed melting point. The air we breathe-a mixture of different gases. It will melt completely at one temperature only.
Water is an example of a pure substance on the other hand salt mixed in water is an example of a mixture. You then end up with pure sand. A pure substance consists only of one element or one compound a mixture consists of two or more different substances not chemically joined together The components of a mixture can usually be.
Pure Substances have sharp melting and boiling point and have standard melting or boiling point contrary to this boiling and melting points of mixtures varies by the proportion of constituents. Thus the composition is the same throughout. Impure substances tend to have a slightly lower melting point than the pure.
To determine if a substance is pure in school laboratories we can check the substances melting or boiling points or use chromatography see Separation Techniques. Pure substances have a sharp melting point but mixtures melt over a range of temperatures. A paper chromatogram can be used to distinguish between pure and impure substances.
Lets consider an example. All compounds are substances but not all substances are compounds. An impure substance is a mixture composed mostly of one pure substance but with trace amounts of other substances which are then called impurities.
Sharpness of the melting point is often used to determine whether a substance is pure or impure. To understand whether ink of any sort is a mixture or a substance first know that substances exist in a pure form while mixtures are combinations of substances. A pure chemical compound is a chemical substance that is composed of a particular set of molecules or ions that are chemically bonded.
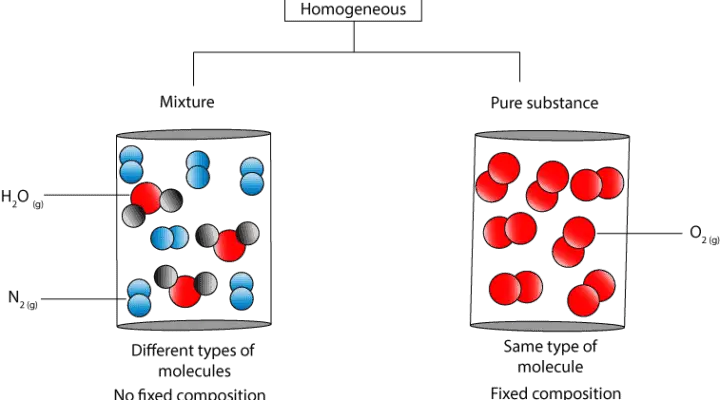
This is the unification of more than one substance incorporated by physical procedures. If it represents a pure substance classify the substance as an element or a compound. Determine whether each molecular diagram represents a pure substance or a mixture.
A pure substance is in the purest form and has no impurities in it while mixture has impurities or is made up of two or more than substances. Chemical and physical properties are constant. If a substance is not chemically pure it is either a heterogeneous mixture or a homogeneous mixture.
In a pure substance the constituents or components are present in fixed ratio or proportion. With impurities the melting point of a substance is affected in two ways. Sharp melting point horizontal flat line on the graph indicates that the substance is pure.
A homogeneous mixture sometimes called a solution is relatively uniform in composition. You can do this by adding water dissolving the salt and then filtering the mixture. Example of substance and mixture include alloys iodized water etc.
Difference Between Pure Substance and Mixture Composition. In turn the substances that make up a mixture are called components. Pure substances cannot be separated into any other kinds of matter while a mixture is a combination of two or more pure substances.
Pure substances are either. If it is pure the substance is either an element or a compound.
This page contains many information about how to determine if a substance is pure or a mixture. Determine whether each molecular diagram represents a pure substance or a mixture. If it represents a pure substance, classify the substance as an element or a compound. If it represents a mixture, classify the mixture as homogeneous or heterogeneous.. Column chromatography: A vertical column is filled with silica gel and solvent, then the mixture is placed on the top and the components pass through to the other end at a time dependent on how attracted they are to the mobile phase. Column chromatography is usually used to separate the components of a mixture during purification.. One of the simplest ways to check the purity of any substance is to compare the substance with a certified pure sample. Even physical comparisons can reveal a lot about the purity of a sample. Visual comparison can reveal the presence of any large impurities, such as dirt or other differently colored impurities.. A pure substance in its purest form does not contain impurities. In a pure substance, the constituents or components are present in fixed ratio or proportion. All pure substances have a uniform characteristic melting and boiling points. In a pure substance, each constituent does not retain its original properties.. Decide whether a substance is chemically pure. If it is pure, the substance is either an element or a compound. If a substance can be separated into its elements, it is a compound. If a substance is not chemically pure, it is either a heterogeneous mixture or a homogeneous mixture..
Videos of how to determine if a substance is pure or a mixture:

Duration: 9:47. Views: 204K views

Duration: 19:12. Views: 249K views
Duration: 11:21. Views: 546 views

Duration: 7:00. Views: 329 views

Duration: 11:15. Views: 4.5K views
Duration: 4:16. Views: 53 views

Duration: 8:55. Views: 585 views

Duration: 13:19. Views: 202 views

Duration: 6:29. Views: 27K views

Duration: 6:57. Views: 33K views

Duration: 6:59. Views: 4.6K views

Duration: 3:37. Views: 1.2K views
You can do this by adding water, dissolving the salt, and then filtering the mixture. You then end up with pure sand. Mixtures can be either homogeneous or heterogeneous: A homogeneous mixture, sometimes called a solution, is relatively uniform in composition; every portion of the mixture is like every other portion.. To determine if a substance is pure in school laboratories, we can check the substance's melting or boiling points or use chromatography (see Separation Techniques). Solids. A pure solid has a constant/fixed melting point. ie. it will melt completely at one temperature only. With impurities, the melting point of a substance is affected in two ways: The melting point is lowered.. 1. A pure substance has only one component Eg: Pure water is a pure substance. It consist of only water molecules. 2. Elements and compounds are both pure substances. It is collection of dissimilar particles that will not undergo a chemical reaction. Mixture: 1. A mixture has variable combinations. Eg: Alcohol-water mixture, both co-exist together. 2. A mixture can be either homogenous or heterogeneous. Sharpness of the melting point is often used to determine whether a substance is pure or impure. An impure substance is a mixture composed mostly of one pure substance but with trace amounts of other substances which are then called impurities. Sharp melting point (horizontal flat line on the graph) indicates that the substance is pure.. a pure substance consists only of one element or one compound a mixture consists of two or more different substances, not chemically joined together The components of a mixture can usually be.... An impure substance is a type of mixture, so melting points can be used to find out if a substance is pure or impure. Impure substances tend to have a slightly lower melting point than the pure.... Homogeneous means the same or uniform throughout. Therefore, a homogeneous substance can either be a mixture or a pure substance. When a homogeneous substance consists of the same type of molecule with fixed and uniform composition throughout, then the substance is a pure substance.. To understand whether ink of any sort is a mixture or a substance, first know that substances exist in a pure form while mixtures are combinations of substances. Pure substances are either.... A pure chemical compound is a chemical substance that is composed of a particular set of molecules or ions that are chemically bonded. Two or more elements combined into one substance through a chemical reaction, such as water, form a chemical compound. All compounds are substances, but not all substances are compounds.. Pure substances include elements an... This chemistry video tutorial focuses on pure substances and mixtures. It's a subtopic of the classification of matter. Pure substances include elements an.... A pure substance is in the purest form and has no impurities in it while mixture has impurities or is made up of two or more than substances. Pure Substances have sharp melting and boiling point and have standard melting or boiling point, contrary to this, boiling and melting points of mixtures varies by the proportion of constituents. Water is an example of a pure substance, on the other hand, salt mixed in water is an example of a mixture.. Substances are often regarded as being 'uncontaminated' to differentiate them from mixtures and they cannot be split into more than one tangible entity. Since they are formed by ionic procedures, they can only be divided by ionic operations equally. Generally, substances can be subdivided into two groups; elements and compounds.. Difference Between Pure Substance and Mixture Composition. Pure substance: Pure substances are made of only one matter; thus the composition is the same throughout. Mixture: Mixtures are made up of several substances that are not chemically bonded. Properties. Pure substance: Chemical and physical properties are constant..
























![[science-101]-pure-substances-and-mixtures](https://i.ytimg.com/vi/JtH8uhOBggQ/hqdefault.jpg)









![[7]-finding-density-of-a-mixture](https://i.ytimg.com/vi/bJsJ-M8B_qY/hqdefault.jpg)






Posting Komentar untuk "How To Determine If A Substance Is Pure Or A Mixture"