Which One Is The Best Way To Determine Whether An Unknown Sample Is A Pure Substance Or A Mixture
Which One Is The Best Way To Determine Whether An Unknown Sample Is A Pure Substance Or A Mixture. Which of the following uses evaporation as a means of separating the. Tasting the given sample c. Non-uniform substance or a mixture. In the center column state whether the material is a pure substance or a mixture.
Which of the following uses evaporation as a means of separating the.
Which one is the best way to determine whether an unknown sample is a pure substance or a mixture. Commonly used to determine whether a reaction is complete. Examples of pure substances include iron steel and water. Liquid A is pure substance while Liquid B is a mixture.
If the material is a pure substance further classify it as either an element or compound in the right column. The colour texture fragrance or taste of all the particles in a pure substance are the same. You can do this by adding water dissolving the salt and then filtering the mixture.
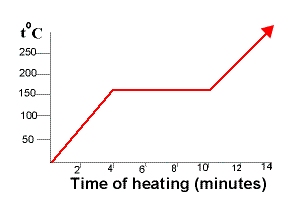
What is the difference between Pure Substance and Mixture. Fill the volumetric flask with your unknown liquid to the fill line. A pure compound always melts and boil at a fixed temperature but a mixture melt and boil at a range of temperature.
Identify the precautions to take with exits in the lab. The melting point is lowered. 16which one is the best way to determine whether an unknown sample is a substance or a mixture.
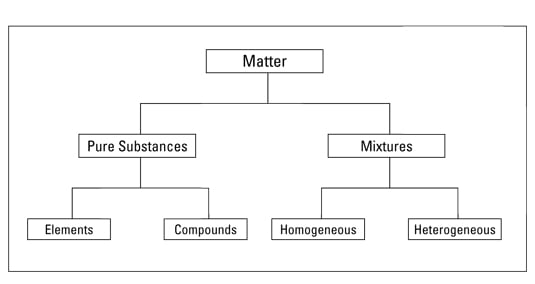
This cannot be separated into any other matter physically. Describe how the new organisms is formed by filling-out the table below. Air is a homogeneous mixture that is often considered to be a pure substance.
Dispose of any excess liquid in the proper waste container. An element is a substance that consists of only one type or kind of atom. There are two methods that can be used to determine the concentration of a substance in a mixture.
Pure substances are substances that are made up of only one kind of particles and has a fixed or constant structure. Pure substances are further classified as elements and compounds. Visually check that the gas cylinder is restrained but keep a safe distance from it.
This works well if you have a fairly pure solution and there is. Mixtures can be either homogeneous or heterogeneous. Determine the mass of the flask plus liquid.
Testing its melting and boiling point. Testing its melting and boiling point 8. From the spectrometric analysis of the unknown sample.
Which is not a technique to separate components of mixture. Tasting the given sample c. To determine if a substance is pure in school laboratories we can check the substances melting or boiling points or use chromatography see Separation Techniques.
Bread is made up of mixtures of different substances. As they are in their purest form they can not be split into different components even by using the chemical or physical processes. Bread is made up of solid and gaseous substances.
Determine the density of the liquid. The pure substances possess similar properties and composition throughout. The liquid if not contaminated can be used for the boiling point determination.
Tasting the given sample C. Observing its physical appearance. With impurities the melting point of a substance is affected in two ways.
Knowing its density B. Aknowing its density Btasting the given sample Cobserving its physical apperance Dtesting its melting and boiling point. Pretty much the same thing as column chromatography except that a small spot of the mixture is placed very close to the bottom of a glass plate covered with silica gel and capillary action pulls the mixture to the top.
If it is a pure. You then end up with pure sand. The first method see below relies upon knowing ε and d and measuring A then you can calculate C from A εCd.
It will melt completely at one temperature only. Select one or more. In the more general sense a pure substance is any homogeneous mixture.
The most accurate means of determining the purity of a substance is through the use of analytical methods. -Locate the exits prior to the start of lab. Which one is the best way to determine whether an unknown sample is a substance or a mixture.
Water and ethanol are pure substances. What is a Pure Substance. The most simple chemical methods include gravimetry and titration.
Which one is the best way to determine whether an unknown sample is a substance or a mixture. Write the entire word in each space to. Every portion of the mixture is like every other portion.
These methods widely used in different industries mostly involve chemical analysis which can pinpoint the presence identity and amount of impurities in the sample. Similarly if the material is a mixture further classify it as homogeneous or heterogeneous in the right column. Which one is the best way to determine whether an unknown sample is a C.
Examine the imagespictures throughout2. Testing its melting and boiling point. Identify the images whether the new individual is formed through sexual or asexual reproductio n3.
17all are techniques which help to seperate components of mixturewhich of these is NOT. Bread is composed of only one substance. Observing its physical appearance.
Tasting the given sample. That is it is matter that appears uniform in appearance and composition no matter how small the sample size. To determine whether the substance is pure or not the chemical method of checking purity is applied.
Agitate by tapping test tube with little finger. Classify each of the materials below. Observing its physical appearance D.
An element is a pure substance as it cannot be broken down or transformed into a new substance even by using some physical or chemical means. Liquids A and B contain two or more atoms that are chemically bonded. A pure solid has a constantfixed melting point.
As mentioned above a pure substance is composed of only one kind of substance. A homogeneous mixture sometimes called a solution is relatively uniform in composition. Thin layer chromatography TLC.
Which one you use depends upon how accurate you wish to be.
This page contains many info about which one is the best way to determine whether an unknown sample is a pure substance or a mixture. Primary School. 8. Which one is the best way to determine whether an unknown sample is a substance or a mixture? A. knowing its density. B. tasting the given sample. C. observing its physical appearance. D. testing its melting and boiling point. 9. Which of the following uses evaporation as a means of separating the.. Thin layer chromatography (TLC): Pretty much the same thing as column chromatography, except that a small spot of the mixture is placed very close to the bottom of a glass plate covered with silica gel, and capillary action pulls the mixture to the top. Commonly used to determine whether a reaction is complete.. 3. What is the difference between Pure Substance and Mixture? What is a Pure Substance. As mentioned above, a pure substance is composed of only one kind of substance. This cannot be separated into any other matter physically. The colour, texture, fragrance or taste of all the particles in a pure substance are the same.. Classify each of the materials below. In the center column, state whether the material is a pure substance or a mixture. If the material is a pure substance, further classify it as either an element or compound in the right column. Similarly, if the material is a mixture, further classify it as homogeneous or heterogeneous in the right column. Write the entire word in each space to. To determine whether the substance is pure or not, the chemical method of checking purity is applied. The pure substances possess similar properties and composition throughout. As they are in their purest form, they can not be split into different components even by using the chemical or physical processes..
Videos of which one is the best way to determine whether an unknown sample is a pure substance or a mixture:

Duration: . Views: 348K views

Duration: 11:39. Views: 495K views
Which one is the best way to determine whether an unknown sample is a substance or a mixture? A. knowing its density. B. tasting the given sample C. observing its physical appearance. D. testing its melting and boiling point. Fill the volumetric flask with your unknown liquid to the fill line. Determine the mass of the flask plus liquid. Determine the density of the liquid. The liquid, if not contaminated, can be used for the boiling point determination. Dispose of any excess liquid in the proper waste container. Figure 1. Agitate by tapping test tube with little finger. Directions:1. Examine the images/pictures throughout.2. Identify the images whether the new individual is formed through sexual or asexual reproductio … n.3. Describe how the new organisms is formed by filling-out the table below.. A pure compound always melts and boil at a fixed temperature but a mixture melt and boil at a range of temperature. 2. From the spectrometric analysis of the unknown sample. If it is a pure.... Pure substances are substances that are made up of only one kind of particles and has a fixed or constant structure. Pure substances are further classified as elements and compounds. An element is a substance that consists of only one type or kind of atom. An element is a pure substance as it cannot be broken down or transformed into a new substance even by using some physical or chemical means.. Visually check that the gas cylinder is restrained but keep a safe distance from it. Identify the precautions to take with exits in the lab. Select one or more: -Locate the exits prior to the start of lab.. 16.which one is the best way to determine whether an unknown sample is a substance or a mixture? A.knowing its density B.tasting the given sample C.observing its physical apperance D.testing its melting and boiling point. 17.all are techniques which help to seperate components of mixture.which of these is NOT? A.condensation B.distillation C.evaporation. The most accurate means of determining the purity of a substance is through the use of analytical methods. These methods, widely used in different industries, mostly involve chemical analysis, which can pinpoint the presence, identity and amount of impurities in the sample. The most simple chemical methods include gravimetry and titration.. Liquid A is pure substance while Liquid B is a mixture. B. Liquids A and B contain two or more atoms that are chemically bonded. I. Bread is solution. II. Bread is composed of only one substance. III. Bread is made up of solid and gaseous substances. IV. Bread is made up of mixtures of different substances. I. Water and ethanol are pure substances. II.. You can do this by adding water, dissolving the salt, and then filtering the mixture. You then end up with pure sand. Mixtures can be either homogeneous or heterogeneous: A homogeneous mixture, sometimes called a solution, is relatively uniform in composition; every portion of the mixture is like every other portion.. To determine if a substance is pure in school laboratories, we can check the substance's melting or boiling points or use chromatography (see Separation Techniques). Solids. A pure solid has a constant/fixed melting point. ie. it will melt completely at one temperature only. With impurities, the melting point of a substance is affected in two ways: The melting point is lowered.. In the more general sense, a pure substance is any homogeneous mixture. That is, it is matter that appears uniform in appearance and composition, no matter how small the sample size. Examples of pure substances include iron, steel, and water. Air is a homogeneous mixture that is often considered to be a pure substance.. 7. Which one is the best way to determine whether an unknown sample is a C. Heterogeneous D. Non-uniform substance or a mixture? A. Knowing its density B. Tasting the given sample c. Observing its physical appearance D. Testing its melting and boiling point 8. Which is not a technique to separate components of mixture? A. Condensation B .... There are two methods that can be used to determine the concentration of a substance in a mixture. Which one you use depends upon how accurate you wish to be. The first method (see below) relies upon knowing ε and d and measuring A, then you can calculate C from A = εCd. This works well if you have a fairly pure solution and there is.




















Posting Komentar untuk "Which One Is The Best Way To Determine Whether An Unknown Sample Is A Pure Substance Or A Mixture"